Background:
Sickle cell disease (SCD) affects approximately 100,000 Americans and causes many morbidities mediated by inflammation. Hydroxyurea (HU), the primary SCD therapy, demonstrates superior outcomes with dose-escalation. While HU induces fetal hemoglobin, it also reduces absolute neutrophil counts (ANC) and inflammation. However, ANC is used to determine if HU can be dose-escalated. Thus, a lower baseline ANC has potential to limit dose escalation and outcomes.
The Duffy null phenotype, ubiquitous among West Africans, reduces ANC through neutrophil egress from the blood into tissues.While this does not increase infection risk, its prevalence and impact on ANC, HU dosing, and outcomes among children with SCD in the U.S. are unknown. Thus, the primary objective of this study was to determine prevalence of Duffy null among children with SCD (any genotype) receiving care and who had erythrocyte phenotyping performed at a large, Midwestern SCD center between 2010-2022. We also aimed to compare baseline ANC of children with SCD and Duffy null and to children with SCD and Duffy positive phenotypes at their one-year hematology visit (overall and by genotype severity). Finally, in those who initiated HU, we aimed to compare ANC, HU dosing, and the frequency of acute healthcare utilization for SCD complications between groups during the first year of HU use.
Methods:
This was a retrospective study of children with SCD. Subjects were identified using a list of those who had phenotyping performed at the center and confirmed to have SCD in their electronic health records. Individuals with Fy a-Fy b- were considered to be Duffy null. Data abstracted included demographics (e.g., age, race) and laboratory studies (e.g., hemoglobin (Hb), ANC). HU prescribing information and emergency department and/or admissions among those who used HU for ≥1year were also recorded. Descriptive statistics (median and interquartile range (IQR)) were used and Chi-squared, Fisher's exact, and Wilcoxon rank sum were used to compare categorical and continuous groups; p-value <0.05 was considered statistically significant.
Results:
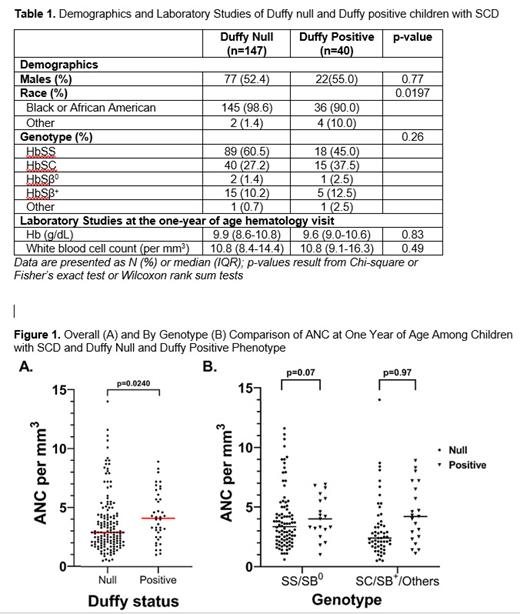
Of 187 children with SCD who had phenotyping; 147 (78.6%) had Duffy null (Table 1) and were compared to Duffy positive children with SCD (Figure 1).
A subset of 72 children with Duffy null and 22 with Duffy positive initiated HU;59 with Duffy null and 15 with Duffy positive remained on HU for ≥1 year. Median HU doses at initiation (p=0.72) and at one year (p=0.35) did not significantly differ between groups. Median ANC at HU initiation was 4.0 mm 3 (IQR 3.3.-5.9) in the Duffy null group and 5.0 mm 3 (IQR 3.2-6.8) in the Duffy positive group but this difference was not significantly different (p=0.21), nor was median ANC at one year between groups (2.0 mm 3, IQR 1.9-4.2 vs. 3.7 mm 3, IQR 2.8-4.9, p=0.07). Median number of acute visits did not differ between the Duffy null (0, IQR 0-1) and Duffy positive (0, IQR 0-1) groups (p=0.65).
Discussion:
We identified that Duffy null, while not ubiquitous, was present in more than three fourths of young children with SCD in the Midwest and was associated with having a lower ANC at one year of life. Given that HU dose-escalation improves outcomes, it was reassuring that those with Duffy null on HU did not have a significantly lower ANC and were not prescribed lower dose HU than Duffy positive children at either point. However, it is notable that these may be because few children in both groups had ANC low enough to preclude HU initiation or escalation based on dosing guidelines and because acute visits were infrequent.
Limitations include the retrospective design, small number of those who initiated and remained on HU for ≥1 year, and short time interval that clinical and laboratory outcomes were compared. Also, it is possible that some did not have phenotyping completed, but standard practice at our center is to complete phenotyping on all with SCD.
In conclusion, Duffy null is highly prevalent in young children with SCD in the U.S. While this does not appear to have an impact on HU dosing of young children with SCD early in their HU course, subsequent longitudinal studies of older children are needed to explore if Duffy null has an impact on longer-term HU dosing and outcomes.
Disclosures
Villella:Vertex CRISPR Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal